PIRS ALERT
19th June 2025
Click to read
PIRS II Update November 2024
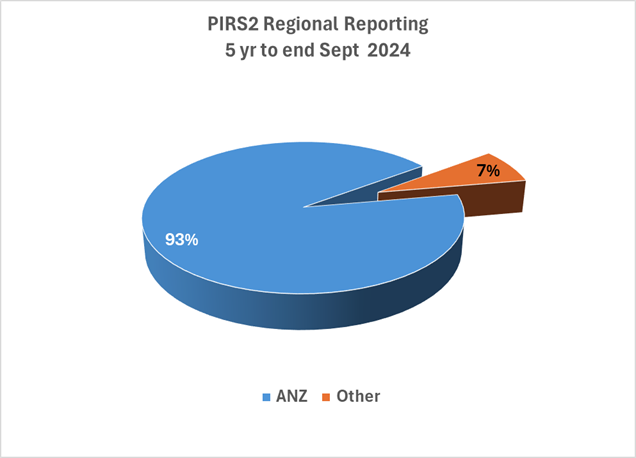
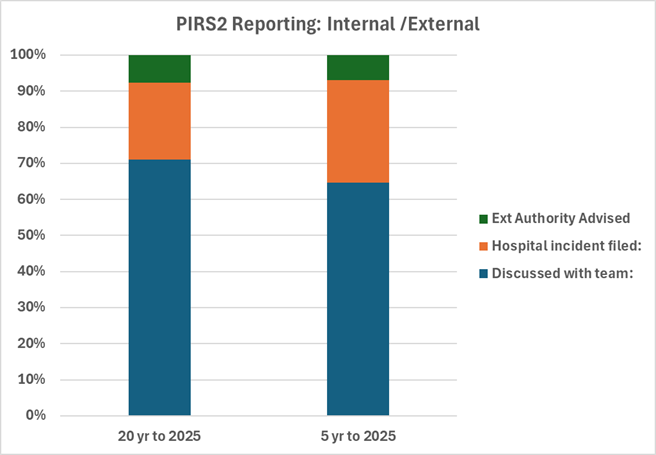
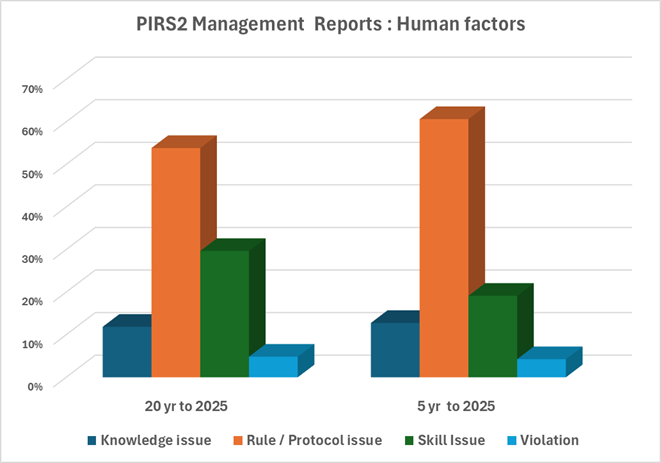
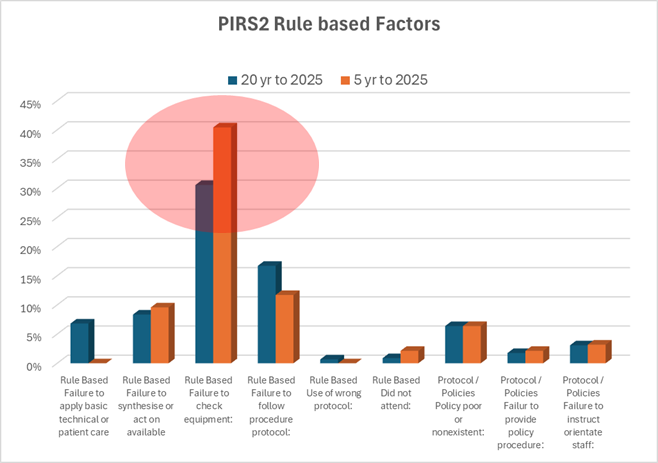
16th December 2024
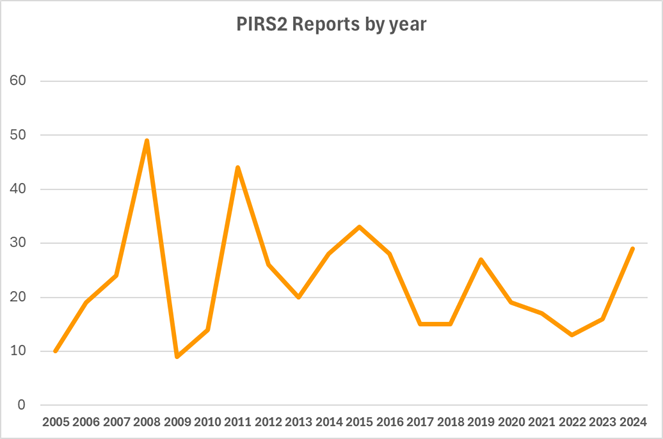
Pleasingly there has been an 80% increase in reports to PIRS 2 for 2024 over 2023 following a decreasing trend in numbers prior to that. At the recent ASCVP Annual Scientific Meeting following a presentation on PIRS2 there was discussion on barriers to reporting. One comment was that editorial commentary could be one such barrier.
The purpose of the editorial commentary – reserved for some reports – is to provide helpful information and promote discussion. The PIRS2 Report submission form Q22 asks for “ Permission to publish edited description in ANZCP Gazette &/or website”. We will add – “and to circulate to the PIRS2 email group”
It is very appropriate to submit a report and answer NO to permission to publish. In a case the deidentified data will simply go into the data base and the relevant categories and human factors used in analyses. Descriptive details will remain embargoed. This may give comfort to those hesitant to report.
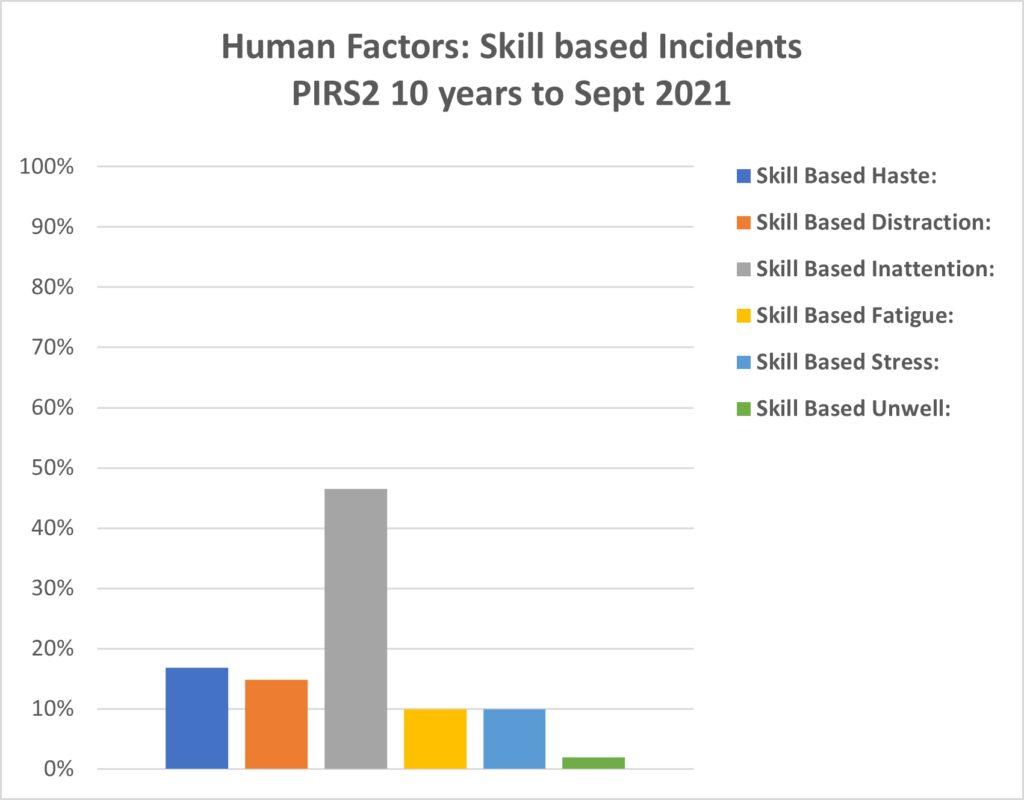
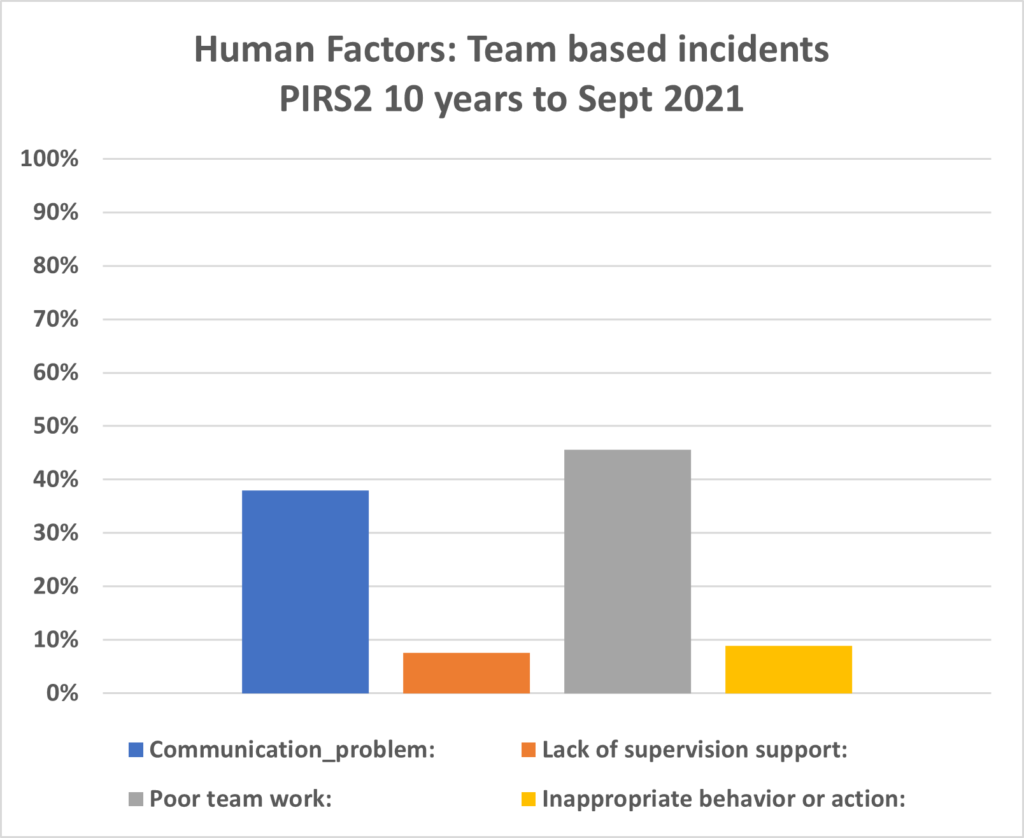
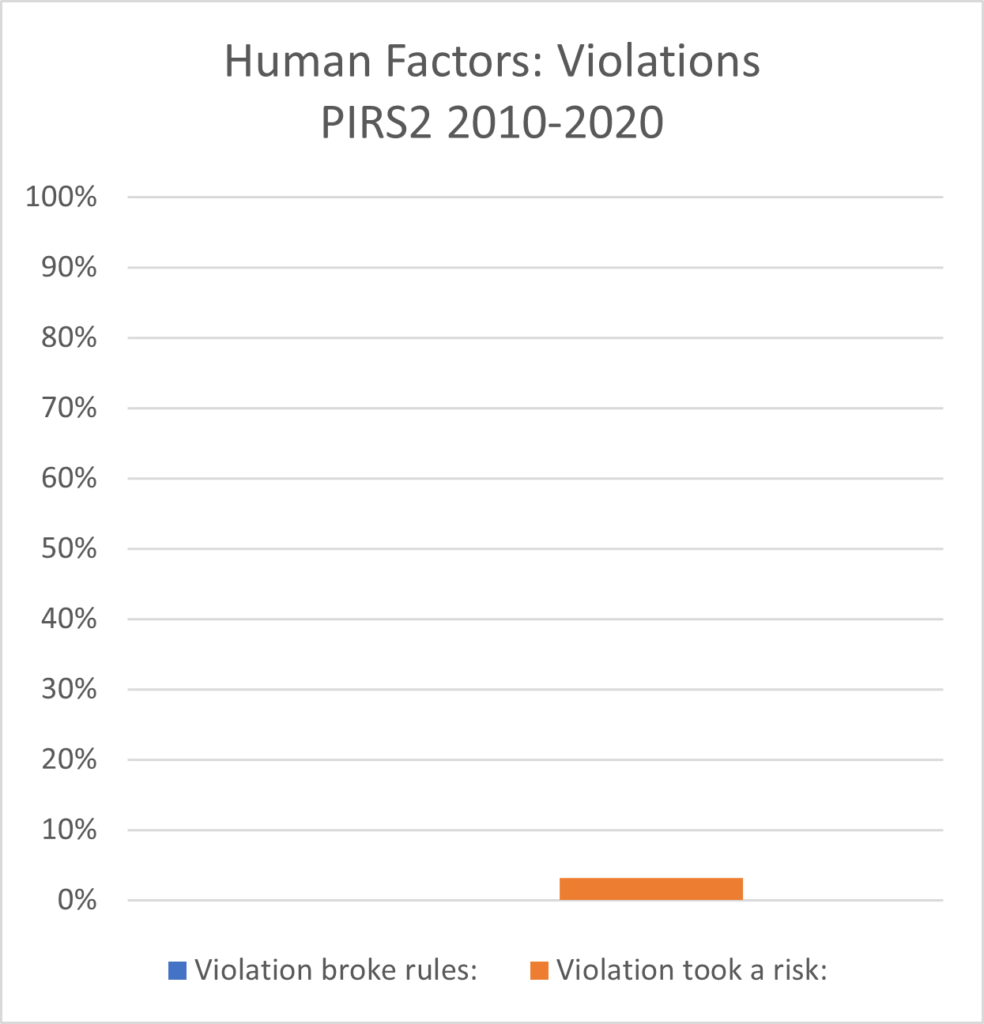
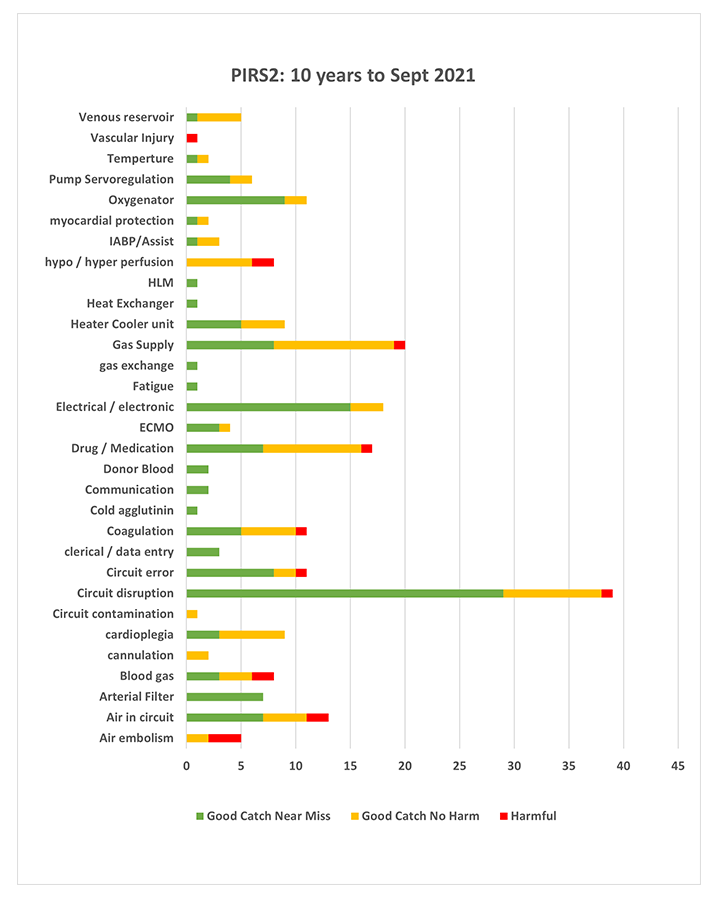
PIRS2 : Human Factors of Reporting Information
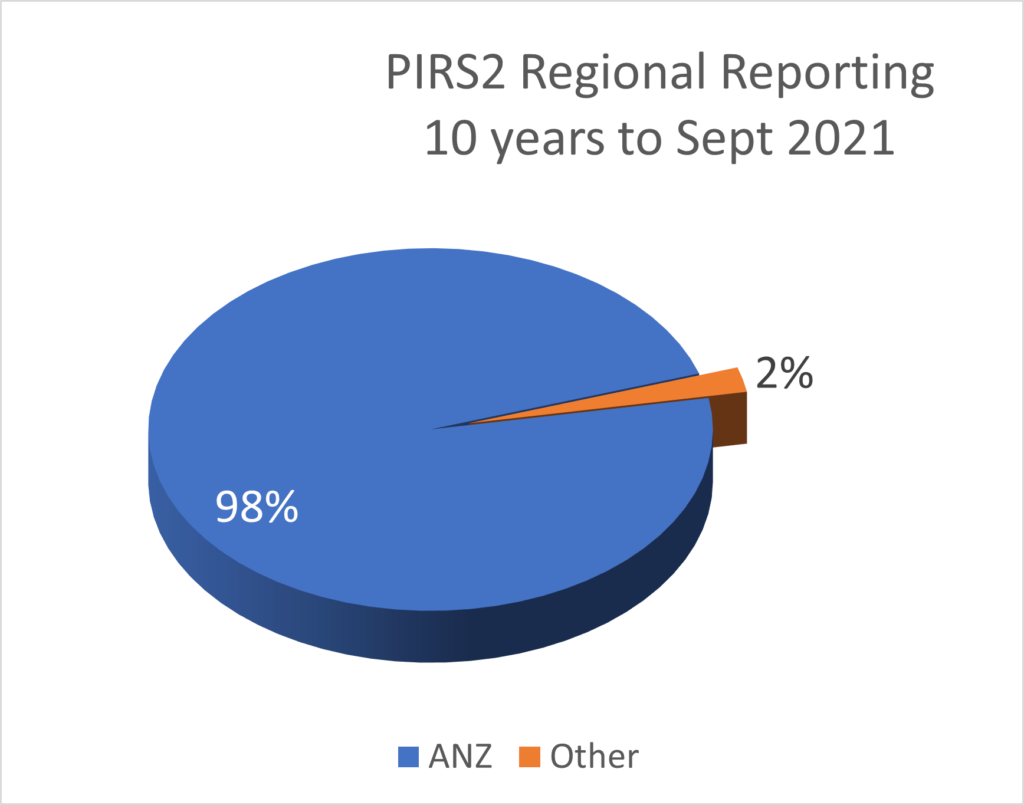
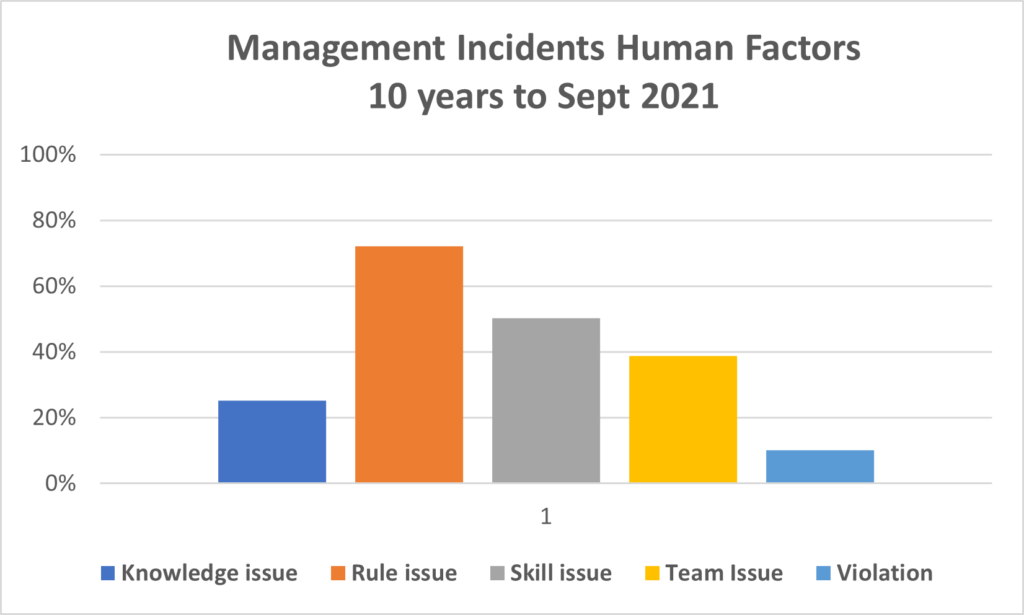
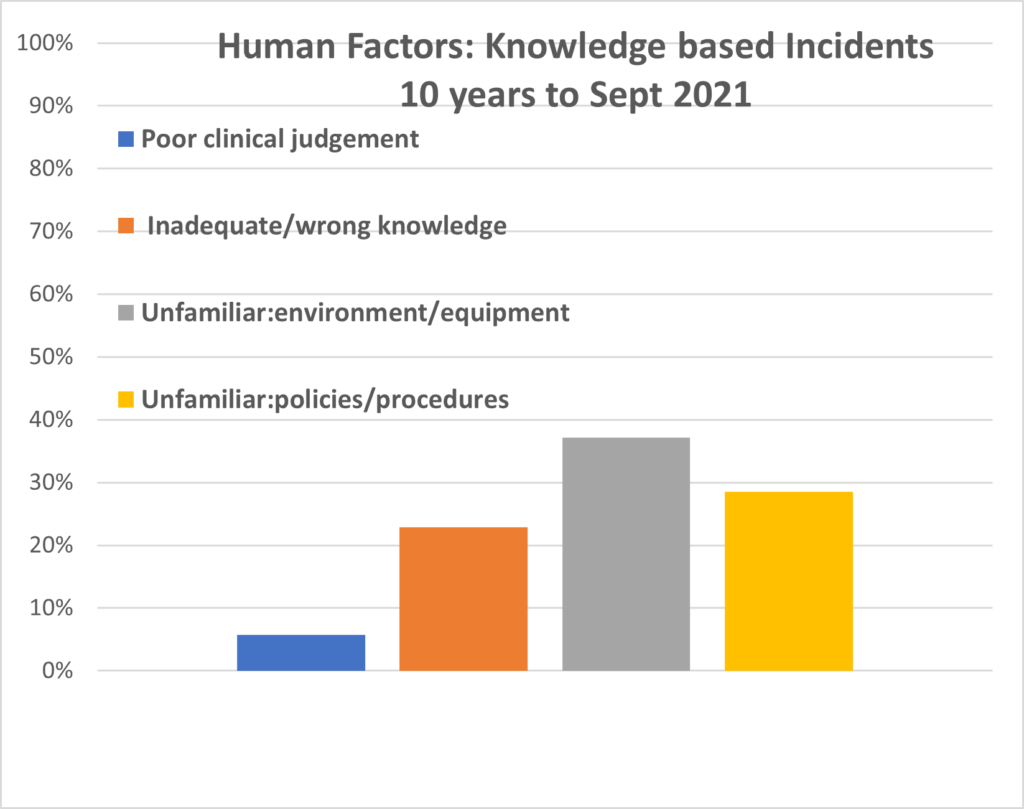
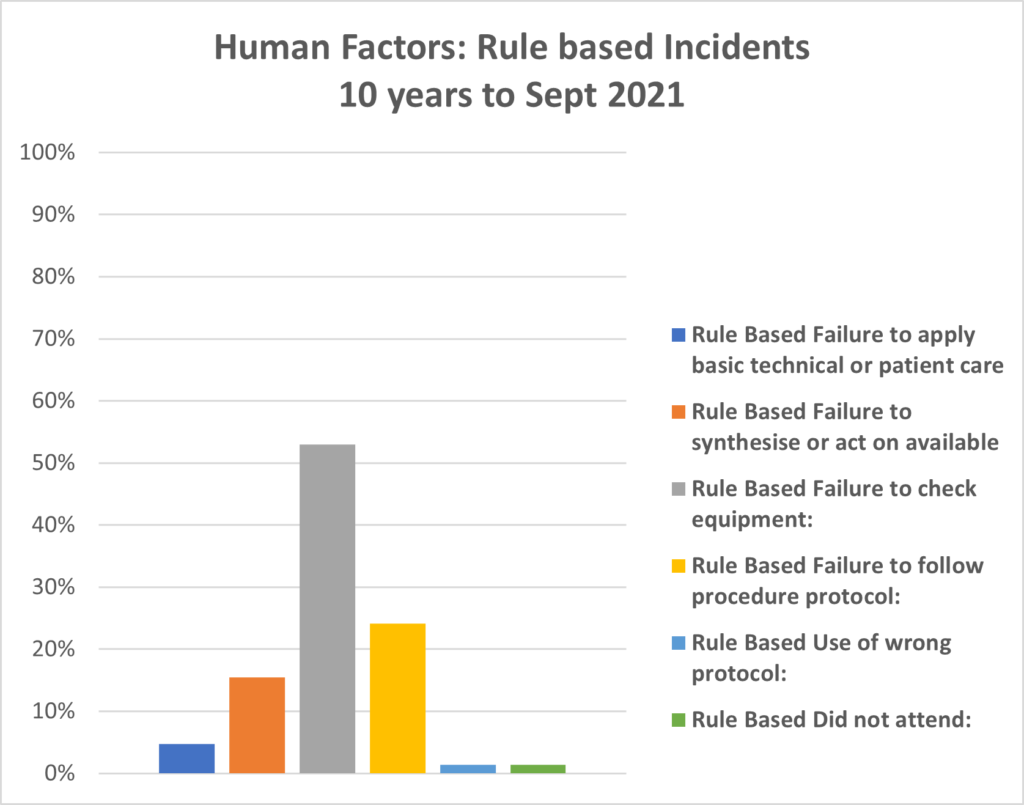
PIRS News 10
June 2020
PIRS News 9
September 2019
PIRS News 8
April 2019
PIRS News 7
April 2018
PIRS News 6
January 2018
PIRS News 5
June 2017
PIRS News 4
May 2017
PIRS News 2
February 2017
PIRS News 1
January 2017
Original Article
Reproduced with permission from JECT
Incident Reporting in Perfusion: Current Perceptions on
PIRS-2
Timothy W. Willcox, CCP;* Robert A. Baker, PhD, CCP^
*Green Lane Cardiothoracic Unit, Auckland City Hospital, Auckland, New Zealand; and Department of Anaesthesiology, School of Medicine, University of Auckland, Auckland, New Zealand; and ^Cardiac and Thoracic Surgery Unit, Flinders Medical Centre, Adelaide, South Australia; College of Medicine and Public Health, Flinders University, Adelaide, South Australia, Australia.
Disconnection of Cobe SMARxT® Tubing from the Venous Outlet of the Terumo Capiox® SX25RX Oxygenator During Cardiopulmonary Bypass
Jane Ottens, BSc, Dip Perf, CCP (Aust),* Robert A. Baker, PhD, Dip, Perf, CCP (Aust),†‡ Andrew J. Sanderson, BSc, Dip, Perf, CCP (Aust),* and Richard F. Newland, BSc, Dip, Perf, CCP (Aust)†
Safety Archive
of the Society of Clinical Perfusion Scientists of Great Britain and Ireland
available here.
Please email admin@anzcp.org for PIRS News 3